The FDA is watching these 14 drugs for serious side effects.
Every 3 months, the FDA reviews and publishes reports of adverse reactions from medications they have received through the FDA Adverse Event Reporting System (FAERS) .The FDA has been posting these quarterly updates since 2007, due to an update to the U.S Food and Drug Administration. Amendments Act that requires the FDA to publish a new list of potential signals of serious risks/new safety information identified every 3 months.
Should I be worried if any prescription is on the list??
The FDA does emphasize that just because a medication appears this list, it doesn’t mean that they have determined that the drug actually carries a risk. Following the quarterly update, the FDA will complete an evaluation of each potential safety issue and make additional announcements if they find anything further.
At the end of the June 2017, the FDA released the list of medications for the first quarter (January-March) that potentially carries serious risks.
So which prescription is the FDA monitoring this quarter???
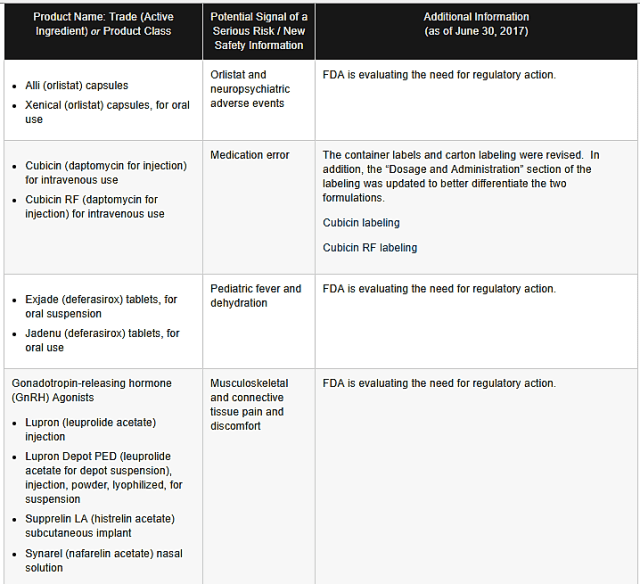
PRODUCT NAME: ALLI (OSLISTAT) CAPSULES, XENICAL (ORLISTAT) CAPSULES FOR ORAL USE
POTENTIAL RISK: Orlistat and neuropsychiatric adverse events
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action
PRODUCT NAME: CUBICIN (DAPTOMYCIN FOR INJECTION) FOR INTRAVENOUS USE, CUBICIN RF (DAPTOMYCIN FOR INJECTION) FOR INTRAVENOUS USE
POTENTIAL RSIK: Medication error
ADDITIONAL INFORMATION: The container labels and carton labeling were revised. In addition the dosage and administration section of the labeling was updated to better differentiate the two formulations. Cubicin labeling, Cubicin RF labeling.
EXJADE (DEFERASIROX) TABLETS, FOR ORAL SUSPENSION,JADENU (DEFERASIROX) TABLETS,FOR ORAL USE
POTENTIAL RISK: Pediatric fever and dehydration
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
Gonadotropin releasing hormone (Gnrh) Agonist
>>LUPRON (LEUPROLIDE ACETATE) INJECTION,LUPRON DEPOT PED (LEUPROLIDE ACETATE FOR DEPOT SUSPENSION),INJECTION,POWDER,LYOPHLIZED,FOR SUSPENSION,SUPPRELIN LA( HISTRELIN ACETATE) SUBCUTANEOUS IMPLANT,SYNAREL(NAFARELIN ACETATE)NASAL SOLUTION
POTENTIAL RISK: Musculoskeletal and connective tissue pain and discomfort
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
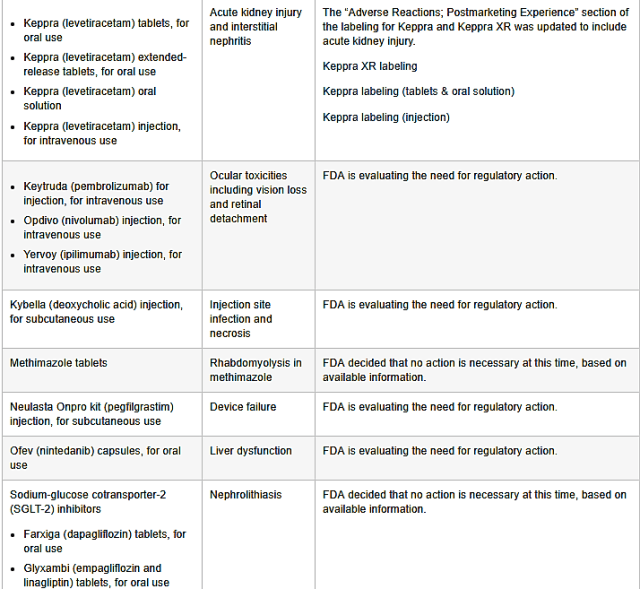
>>KEPPRA (LEVETIRACETAM) TABLETS FOR ORAL USE
>>KEPPRA (LEVETIRACETAM) EXTENDED RELEASE TABLETS FOR ORAL USE
>>KEPPRA (LEVETIRACETAM) ORAL SOLUTION
R>>KEPPRA (LEVETIRACETAM) INJECTION, FOR INTRAVENOUS USE
POTENTIAL RISK: Acute kidney injury and interstitial nephritis
ADDITIONAL INFPRMATION: The adverse reactions, post marketing experience section of the labeling for Keppra and keppra XR was updated to include acute kidney injury.
KEYTRUDA (PEMBEOLIZUMAB) for injection for intravenous use, OPDIVO (NIVOLUMAB) for injection, for intravenous use, YERVOY (IPILIMUMAB) injection, for intravenous use.
POTENTIAL RISK: Ocular toxicities including vision loss and retinal detachment
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
KYBELLA (DEOXYCHOLIC ACID) injection, for subcutaneous use
POTENTIAL RISK: Injection site infection and necrosis
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
METHIMAZOLE TABLETS:
POTENTIAL RISK: Rhabdomyolysis in methimazole
ADDITIONAL INFORMATION: FDA decided that no action is necessary at this time, based on available information.
NEULASTA ONPRO KIT (PEGFILGRASTIM) injection, for subcutaneous use
POTENTIAL RISK: Device failure
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
OFEV (NINTEDANIB) capsules, for oral use
POTENTIAL RISK: Liver dysfunction
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
SODIUM GLUCOSE COTRANSPORTER- 2(SGLT-2) INHIBITORS
>>>FARXIGA (DAPAGLIFLOZIN) tablets, for oral use
>>>GLYXAMBI (EMPAGLIFLOZIN AND LINAGLIPTIN) tablets, for oral use
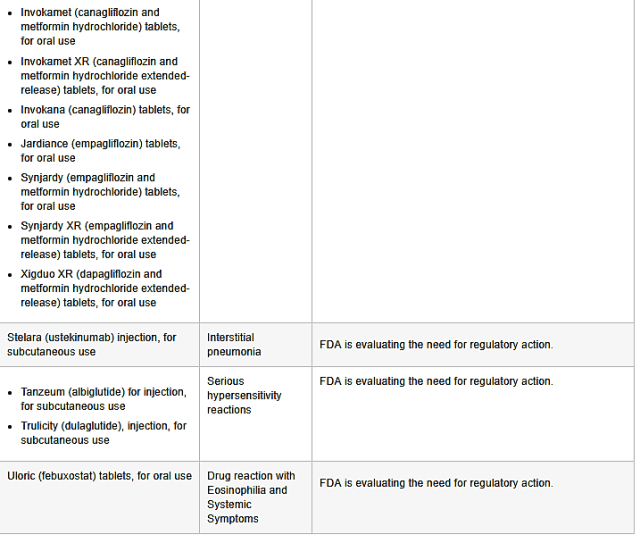
>>>INVOKAMET XR (CANAGLIFLOZIN AND METFORMIN HYDROCHLORIDE EXTENDED-RELEASE) tablets, for oral use
>>>INVOKANA (CANAGLIFLOZIN) tablets, for oral use
>>>JARDAINCE (EMPAGLIFLOZIN) tablets, oral use
>>>SYNJARDY (EMPAGLIFLOZIN AND METFORMIN HYDROCHLORIDE) tablets, for oral use
>>>SYNJARDY XR (EMPAGLIFLOZIN and METFORMIN HYDROCHLORIDE EXTENDED-RELEASE) tablets, for oral use
>>>XIGDUO XR (DAOAGLIFLOZIN and METFORMIN HYDROCHLORIDE EXTENDED-RELEASE) tablets, for oral use
POTENTIAL RISK: Nephrolithiasis
ADDITIONAL INFORMATION: FDA decided that no action is necessary at this time, based on available information.
STELARA (USTEKINUMAB) injection, for subcutaneous use
POTENTIAL RISK: Interstitial pneumonia
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
>>>TANZEUM (ALBIGLUTIDE) for injection, foe subcutaneous use
>>>TRULICITY (DULAGLUTIDE) injection, for subcutaneous use
POTEBTIAL RISK: Serious hypersensitivity reactions.
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory action.
ULORIC (FEBUXOSTAT) tablets, for oral use
POTENTIAL RISK: Drug reaction with Eosinophilia and systemic symptoms.
ADDITIONAL INFORMATION: FDA is evaluating the need for regulatory actions.
Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS): January – March 2017
What is the FDA doing about all of these reports??
Unless otherwise noted, the FDA is currently evaluating the need for further action on all of the medications on the list. What should I do if I take one of these medications?
First, I don’t stop taking your prescription without speaking to your doctor, this can cause more problems than it solves. If you have any concerns or if you think you are experiencing one of these side effects, talk to your pharmacist.
How can we report a problem with a drug to FDA??
IF you need to report a serious problem with a medication to the FDA, you can do so through their MedWatch website. The MedWatch allows you to voluntarily report a serious adverse event, product quality problem, product use error, or therapeutic in equivalence/failure that you suspect is associated with the use of an FDA-regulated drug, biologic, medical device, dietary supplement or cosmetic. You can report suspected counterfeit medical products here as well.







